#api impurity
Explore tagged Tumblr posts
Text
The Role of 4-Desdimethylamine 4-Oxo Omadacycline in Antibiotic Development
The pharmaceutical industry continues to innovate in the fight against antibiotic resistance, and 4-Desdimethylamine 4-Oxo Omadacycline stands out as a pivotal intermediate in this mission. As a structural precursor in the synthesis of omadacycline, this compound plays a central role in enabling the production of next-generation antibiotics with improved efficacy and resistance profiles.
What is 4-Desdimethylamine 4-Oxo Omadacycline?
4-Desdimethylamine 4-Oxo Omadacycline is a chemically engineered intermediate designed for use in the synthesis of omadacycline, a novel aminomethylcycline antibiotic. Its molecular structure is critical in allowing modifications that enhance antibacterial activity while maintaining a broad spectrum of action, even against drug-resistant bacteria.
This compound is frequently used in:
Pharmaceutical R&D pipelines
Structure-activity relationship (SAR) studies
Intermediate stages of GMP-grade active pharmaceutical ingredient (API) manufacturing
Explore this product: 4-Desdimethylamine 4-Oxo Omadacycline — Aquigen Bio
Related Omadacycline Intermediates & Impurities
In addition to 4-Desdimethylamine 4-Oxo Omadacycline, other related compounds serve important functions in synthesis, impurity profiling, and pharmacological testing. These include:
1. Dihydro Omadacycline Impurity
This impurity is commonly encountered in omadacycline synthesis and is crucial for analytical method development and stability testing. It plays a vital role in ensuring regulatory compliance through impurity profiling.
2. N-(Hydroxymethyl) Omadacycline
Often used in the synthesis of modified tetracycline derivatives, this compound helps scientists optimize structural analogues and improve pharmacokinetic profiles.
3. N-Methyl Omadacycline
This methylated analogue is studied for its structural influence on omadacycline’s antibacterial spectrum and resistance stability. It can also be useful in toxicity and metabolic pathway studies.
Why These Compounds Matter
Each of these compounds, including 4-Desdimethylamine 4-Oxo Omadacycline, contributes uniquely to the research and development of new antibiotics:
Enhancing efficacy against resistant pathogens
Enabling structural modifications for drug optimization
Supporting analytical research through impurities and metabolites
Together, they form a toolkit for scientists working to stay ahead in the global battle against antimicrobial resistance.
Sourcing from a Trusted Supplier
For researchers and manufacturers seeking reliable, high-purity antibiotic intermediates and impurities, Aquigen Bio offers:
GMP-compliant sourcing
Consistent batch quality
Responsive technical support
Whether you’re scaling synthesis or conducting early-stage research, Aquigen Bio ensures access to compounds that meet both scientific and regulatory demands.
Final Thoughts
As antibiotic innovation accelerates, key intermediates like 4-Desdimethylamine 4-Oxo Omadacycline, along with related compounds like Dihydro Omadacycline Impurity, N-(Hydroxymethyl) Omadacycline, and N-Methyl Omadacycline, will continue to empower breakthroughs in drug discovery and development.
Looking for a quote or COA? Visit Aquigen Bio or explore their Contact Page to connect with a specialist.
0 notes
Text
Alentris Research Private Limited
At Alentris Research Private Limited, we are dedicated to advancing the field of impurity science since our founding in 2019. Our experienced team provides solutions for pharmaceutical, biopharmaceutical, and chemical companies seeking expertise in API Impurities, Working Standards, Analytical Standards, Degradation Impurities, Genotoxic Impurities, Organic Impurities, and satisfying Regulatory Requirements for impurity testing and identification. Leveraging state-of-the-art analytical instrumentation and methodologies, we specialize in the Isolation and Characterisation of both known and Unknown Impurities, enabling the Discovery of Impurities to support development, improve quality, and ensure safety. Get in touch to learn more about our end-to-end impurity management services.
With expertise in Impurity Research, our scientific team specializes in the custom Synthesis of Impurity standards including Nitrosamines Impurities, Oncology Impurities, and Peptide Impurities. We are dedicated to supporting pharmaceutical companies through expert Impurity services for drug development, quality control, and regulatory compliance. Contact us to learn more about our capabilities in impurity management.
We offer Force Degradation studies to assess impurity profiles and meet EP and USP Impurities guidelines. Our capabilities include Photolytic Degradation Impurity, Thermal Degradation Impurity, Oxidative Degradation Impurity, and Acid-Alkaline Degradation Impurity testing. For pharmaceutical companies, we provide impurity analysis and identification in drug substances, Formulation Impurities, degradation products, and more. Contact us to learn about our impurity management services for API and drug development.
1 note
·
View note
Text
High-Quality Atorvastatin Impurity Standards for Accurate Pharmaceutical Research
Atorvastatin is one of the most widely prescribed statins, primarily used to lower cholesterol and prevent cardiovascular diseases. As a crucial component in generic and branded formulations, atorvastatin must be tested with the highest standards of precision and accuracy. At Aquigen Bio, we offer an advanced range of atorvastatin impurity standards to support pharmaceutical development, quality control, and regulatory compliance.
Why Are Atorvastatin Impurity Standards Important?
In drug development and production, the identification and quantification of impurities play a critical role in ensuring safety, efficacy, and compliance with international guidelines such as ICH and FDA. Impurity profiling of atorvastatin helps manufacturers meet stringent quality requirements, providing confidence in the drug’s therapeutic effectiveness.
Aquigen Bio’s impurity standards are synthesized and characterized with high purity and traceability, ensuring accurate results in analytical testing.
Explore Our Range of Atorvastatin Impurity Standards
Our collection of atorvastatin-related impurity standards includes rare and stable isotope-labeled compounds to support bioanalytical and pharmacokinetic studies.
1. (3R,5S)-Atorvastatin Calcium salt
This stereospecific calcium salt form of atorvastatin plays a critical role in chiral impurity testing and stereoisomer profiling. Its precise structure helps ensure the enantiomeric purity of the active pharmaceutical ingredient (API).
2. 2-Hydroxy Atorvastatin D5
This deuterated metabolite is valuable in mass spectrometry applications. The presence of five deuterium atoms enhances sensitivity and precision in LC-MS/MS quantification, making it an ideal internal standard in pharmacokinetic and metabolic stability studies.
3. 2-Hydroxy Atorvastatin D5 Disodium Salt
The disodium salt form improves solubility and stability, offering a reliable reference standard for bioavailability and dissolution testing. It’s especially useful in developing robust analytical methods for complex matrices.
Why Choose Aquigen Bio?
Aquigen Bio stands out as a trusted partner for high-purity impurity standards. Here’s what makes us different:
GMP-compliant synthesis
Certificate of Analysis (CoA) with detailed characterization data
Global shipping with temperature-controlled logistics
Customized solutions for R&D and commercial needs
Whether you’re developing a generic atorvastatin formulation or optimizing quality control processes, our range of impurity standards supports every stage of pharmaceutical analysis.
0 notes
Text
Exploring the Potential of Nicotine USP in New Pharmaceutical Formulations By Prism Industries Pvt. Ltd.
In the ever-changing world of healthcare and nicotine replacement therapies, nicotine USP has become a key player in crafting modern pharmaceutical solutions. With its rigorous purity standards, nicotine USP is leading the charge for safer and more effective options in products like gums, patches, lozenges, and oral strips. As a top manufacturer of nicotine USP, Prism Industries Pvt. Ltd. is at the cutting edge of innovation in the API manufacturing sector, providing dependable and compliant solutions to pharmaceutical brands around the globe.
This article delves into the growing applications, advantages, and formulation considerations of nicotine USP, underscoring its increasing significance in contemporary drug development.
Understanding Nicotine USP and Its Pharmaceutical Value
Nicotine USP is high-purity nicotine that meets the standards set by the United States Pharmacopeia. These strict guidelines guarantee the safety, effectiveness, and consistency of nicotine used in medicinal products.
Unlike crude or synthetic alternatives, nicotine USP is refined, rigorously tested, and validated for use in a variety of therapeutic formulations. This makes it the go-to choice for nicotine USP manufacturers worldwide, particularly for applications in nicotine replacement therapy (NRT), cognitive enhancement research, and smoking cessation initiatives.
Applications of Nicotine USP in Pharmaceutical Formulations
1. Nicotine Gums and Lozenges
One of the most common applications of nicotine USP is in chewing gums and lozenges aimed at helping people cut back on their tobacco use. These products deliver a controlled amount of nicotine, making it easier for the body to gradually reduce its dependence on cigarettes.
2. Nicotine Patches
Transdermal patches that contain nicotine USP provide a steady release of nicotine, helping to keep plasma levels consistent. Healthcare professionals often recommend these patches as part of a comprehensive smoking cessation plan.
3. Sublingual Tablets and Oral Strips
Fast-dissolving oral strips and tablets utilize nicotine USP to offer quick relief without affecting the respiratory system. This method is becoming increasingly popular due to its ease of use and effectiveness.
4. Inhalers and Nasal Sprays
Nicotine USP is also found in measured-dose inhalers and nasal sprays, providing options for those who want a smoking-like experience without the harmful tar and toxins.
When it comes to the benefits of Nicotine USP in new formulations, there are several key points to consider:
1. High Purity for Enhanced Safety
Nicotine USP meets strict pharmacopeial standards, which means it’s free from impurities that could be harmful. This ensures that the product is clean and safe for therapeutic use.
2. Consistent Dosage Delivery
In the pharmaceutical world, consistency is crucial. Nicotine USP guarantees uniform dosage, which is vital for achieving reliable patient outcomes and staying compliant with regulations.
3. Regulatory Acceptance Worldwide
Products made with nicotine USP have a better chance of gaining approval from health authorities around the globe, thanks to their high-quality standards. This makes it easier to export and market these products internationally.
4. Versatility in Formulation
Whether you’re looking at patches, gum, or strips, nicotine USP provides the flexibility needed for various formulations without sacrificing stability or bioavailability.
Role of Nicotine USP Manufacturers in Quality Assurance
As a prominent player in the nicotine USP manufacturing scene, Prism Industries Pvt. Ltd. adheres to globally recognized quality systems to produce pharmacopeial-grade nicotine. With our state-of-the-art infrastructure, quality testing labs, and deep regulatory expertise, we consistently meet the needs of clients across the pharmaceutical landscape.
We focus on:
- Precision extraction and purification techniques
- GMP-certified manufacturing processes
- Analytical testing to ensure purity, potency, and shelf-life stability
- Customized packaging to prevent degradation
This commitment positions Prism Industries Pvt. Ltd. as a reliable name in the API manufacturing industry and a trusted supplier of nicotine USP for both established and emerging pharmaceutical brands.
Challenges in Formulating with Nicotine USP
1. Sensitivity to Light and Moisture
Nicotine USP is quite sensitive to its surroundings, which means it needs to be stored in air-tight, light-proof packaging to keep its effectiveness intact.
2. Dose-Dependent Toxicity
While nicotine USP is generally safe at therapeutic doses, higher amounts can be harmful. It's crucial for formulators to be precise when blending and distributing it across different delivery systems.
3. Taste Masking in Oral Products
When it comes to gums and lozenges, additional excipients might be necessary to cover up the natural bitterness of nicotine USP, all while ensuring proper absorption.
By collaborating with seasoned nicotine USP manufacturers like Prism Industries Pvt. Ltd., these hurdles can be tackled effectively through the application of formulation science and quality control expertise.
The Growing API Manufacturing Industry and Nicotine USP
India is emerging as a key player in the global API manufacturing scene, with reputable companies like Prism Industries Pvt. Ltd. leading the charge in exports and innovation.
Why India Excels in Nicotine USP Production:
Access to top-notch raw tobacco sources
Cost-effective yet compliant production techniques
A skilled workforce with extensive pharmaceutical knowledge
A solid reputation among international regulatory authorities
Prism Industries Pvt. Ltd. not only takes advantage of these national strengths but also consistently upgrades its facilities and processes to keep pace with changing global standards.
Regulatory and Market Outlook for Nicotine USP
The demand for nicotine USP is projected to see significant growth over the next decade, driven by:
A surge in the use of smoking cessation aids around the world
Growing awareness of harm-reduction alternatives
An increase in therapeutic research exploring cognitive and neurological applications of nicotine
Stricter regulations on tobacco, leading to a push for safer options
Governments and regulatory agencies are now advocating for the use of verified nicotine USP instead of raw or synthetic alternatives, encouraging pharmaceutical companies to partner with certified suppliers.
Why Choose Prism Industries Pvt. Ltd. for Nicotine USP
Prism Industries Pvt. Ltd. stands at the forefront of nicotine USP production and supply. With years of experience in the API manufacturing sector, we deliver unparalleled quality, scalability, and compliance.
What Makes Us Unique:
Facilities certified by WHO-GMP and ISO
Thorough documentation and Certificates of Analysis with every shipment
Consistent quality from batch to batch, verified by third parties
A dedicated R&D team ready to assist with customized formulation needs
When you choose to work with Prism Industries Pvt. Ltd., you’re not just getting a supplier—you’re gaining a strategic partner committed to developing reliable nicotine-based formulations for the future.
The world of pharmaceuticals is changing at a rapid pace, and nicotine USP is emerging as a key player in creating safe, effective, and innovative drug delivery systems. Thanks to its exceptional purity and versatility, nicotine USP helps formulators meet the growing global demand for harm-reduction products and therapeutic alternatives.
By partnering with experienced nicotine USP manufacturers like Prism Industries Pvt. Ltd., pharmaceutical companies can tackle formulation challenges head-on and provide impactful solutions that are backed by regulatory compliance and scientific credibility. As leaders in the api manufacturing industry, we are dedicated to fostering the future of nicotine innovation through our commitment to quality, safety, and excellence.
When it comes to your next-generation nicotine USP needs, choose Prism Industries Pvt. Ltd.—where pharmaceutical vision aligns with manufacturing expertise.
#nicotine_usp#nicotine_usp_manufacturers#pure_nicotine_manufacturers#nicotine_Manufacturer#nicotine_manufacturers
0 notes
Text
Best Diphenyl Acetonitrile Manufacturer in Philippines

Introduction
The pharmaceutical and chemical industries in the Philippines are expanding steadily, with a growing demand for high-purity chemical intermediates. One such important compound is Diphenyl Acetonitrile, widely used in the synthesis of pharmaceutical intermediates, dyes, agrochemicals, and specialty chemicals. Choosing the right manufacturer is crucial to ensure quality, safety, and consistency in production.
If you are searching for the best Diphenyl Acetonitrile manufacturer in the Philippines, Dolphin Pharmaceutical stands out as the trusted name. With a proven track record in chemical and drug manufacturing, Dolphin Pharmaceutical is a reliable pharmaceutical drug manufacturer in Philippines, offering exceptional quality and customer support.
What is Diphenyl Acetonitrile?
Diphenyl Acetonitrile (DPAN), chemically known as (C6H5)2CHCN, is a versatile organic intermediate. It appears as a white to pale yellow crystalline solid and is used in multiple applications:
Pharmaceutical Intermediates: DPAN plays a role in the synthesis of various active pharmaceutical ingredients (APIs).
Agrochemical Industry: Used in the production of herbicides and pesticides.
Dye and Pigment Manufacturing: An important component in colorants.
Specialty Chemicals: Acts as a base compound for many high-value chemical derivatives.
Its applications demand high purity, controlled processing, and dependable quality—all of which Dolphin Pharmaceutical delivers.
Why Quality Manufacturing Matters
In pharmaceuticals and chemicals, even slight impurities can impact product safety, stability, and performance. Hence, selecting a manufacturer that provides:
High purity Diphenyl Acetonitrile
Batch-to-batch consistency
Stringent quality control
Compliant packaging and handling
Reliable and on-time delivery
is essential. Dolphin Pharmaceutical ensures all of these and more, meeting both domestic and international quality standards.
Dolphin Pharmaceutical – A Trusted Manufacturer
Dolphin Pharmaceutical has earned a reputation as a reliable pharmaceutical drug manufacturer in Philippines, providing clients with high-grade Diphenyl Acetonitrile for various industrial uses.
Our commitment to quality, safety, and customer satisfaction has helped us become a preferred supplier for leading businesses across the country.
Key Strengths:
GMP-Certified Manufacturing Units: Our facilities meet strict quality and hygiene standards.
Robust Quality Assurance (QA): Every batch undergoes rigorous lab testing.
Experienced Team: Our chemical engineers and technicians ensure accuracy and compliance.
Custom Packaging: Delivered in safe, sealed containers as per customer requirements.
Nationwide Supply Chain: Reliable logistics for timely deliveries across the Philippines.

Role in Pharmaceutical Industry
In the pharmaceutical sector, Diphenyl Acetonitrile is used in the synthesis of drugs requiring a nitrile group as a functional precursor. The compound's reactivity makes it ideal for creating new formulations and intermediates.
As a pharmaceutical drug manufacturer in Philippines, Dolphin Pharmaceutical understands the specific quality and documentation standards required by the pharma sector.
We provide:
Complete product traceability
Material safety data sheets (MSDS)
COA (Certificate of Analysis)
Customized lot sizes based on order needs
This integrated approach gives clients confidence in both product safety and regulatory compliance.
Environmental and Safety Commitment
Producing chemicals like Diphenyl Acetonitrile requires careful adherence to environmental standards. Dolphin Pharmaceutical takes its environmental responsibilities seriously and implements:
Advanced waste treatment systems
Emissions control technology
Zero-discharge wastewater systems
Safe storage and transport protocols
This ensures our operations not only meet legal requirements but also protect the environment and surrounding communities.
Industries We Serve
While Diphenyl Acetonitrile is widely used in pharmaceuticals, its applications extend to other important industries, including:
Agrochemicals: For synthesizing crop protection products
Textiles and Dyes: As an intermediate in colorants and pigment formulations
Specialty Chemicals: For laboratory and industrial synthesis
Research and Development: Universities and institutions use DPAN in experimental chemistry
Our consistent quality and flexibility in order sizes make us a go-to supplier for startups, labs, and large-scale industries alike.
Why Choose Dolphin Pharmaceutical
Dolphin Pharmaceutical is more than just a Diphenyl Acetonitrile manufacturer. As a full-spectrum pharmaceutical drug manufacturer in Philippines, we offer the experience, infrastructure, and certifications needed to produce high-quality compounds.
Here’s why clients choose us:
Over a decade of experience in pharmaceutical and chemical manufacturing
Comprehensive product portfolio including APIs and intermediates
Full compliance with international and national quality norms
Client-centric approach with personalized service
Affordable pricing with no compromise on quality
Conclusion
In a competitive and regulated industry, selecting the right supplier for your raw materials is crucial. Dolphin Pharmaceutical’s focus on quality, transparency, and service makes it the best Diphenyl Acetonitrile manufacturer in Philippines.
Our deep knowledge as a pharmaceutical drug manufacturer in Philippines allows us to deliver products that meet the strictest quality standards.
#Diphenyl Acetonitrile Manufacturer in Philippines#Diphenyl Acetonitrile supplier in Philippines#Diphenyl Acetonitrile exporter in Philippines
0 notes
Text
Global Dabigatran Manufacturer
Understanding Dabigatran Manufacturing
In the ever-evolving landscape of pharmaceuticals, anticoagulants have emerged as crucial tools in preventing life-threatening conditions such as strokes and venous thromboembolism. Among these, dabigatran stands out as a widely used oral direct thrombin inhibitor. Though commonly prescribed, the manufacturing of dabigatran is a complex, tightly regulated process that blends high-end chemistry with rigorous safety standards. This blog explores the process of dabigatran manufacturing, highlighting key aspects like synthesis, formulation, quality control, and regulatory compliance.
What is Dabigatran?
Dabigatran is an oral anticoagulant that functions by inhibiting thrombin, a key enzyme in the blood-clotting process. It is typically used to prevent stroke and systemic embolism in patients with non-valvular atrial fibrillation, and also for the treatment and prevention of deep vein thrombosis (DVT) and pulmonary embolism (PE).
It is usually administered in its prodrug form, dabigatran etexilate, which becomes active after metabolism in the liver.
Manufacturing Dabigatran: From Lab to Tablet
Manufacturing dabigatran involves a sophisticated process that includes chemical synthesis, formulation, and packaging, all under stringent quality assurance protocols.
1. Active Pharmaceutical Ingredient (API) Synthesis
The core of any pharmaceutical product is the Active Pharmaceutical Ingredient (API). Dabigatran’s API, dabigatran etexilate mesylate, is synthesized through a series of multi-step organic reactions. These reactions involve precise control of chemical conditions such as pH, temperature, and solvent choice.
The synthesis pathway is complex and typically starts with hydroxybenzamidine derivatives, which undergo reactions including esterification, amidation, and salt formation to yield the final mesylate salt. Each intermediate stage must be meticulously purified and analyzed to ensure the desired stereochemistry and chemical stability are maintained.
Key considerations during API synthesis include:
Purity: Impurities must be controlled to meet international pharmacopeia standards.
Yield: Efficient chemical processes are essential to keep production economically viable.
Environmental and Worker Safety: Proper handling of solvents, reagents, and byproducts is critical to minimize health and environmental risks.
2. Formulation of the Final Dosage Form
Once the API is synthesized and verified, the next step is the development of the oral solid dosage form, typically a capsule. Dabigatran etexilate has low bioavailability and is sensitive to moisture and acidity, which makes its formulation particularly challenging.
Key elements in the formulation phase include:
Pellet Technology: Dabigatran is often formulated into small pellets coated with functional layers that protect the drug and control its release.
Enteric Coating: This prevents degradation in the stomach and ensures the drug is absorbed in the intestine.
Excipients: These inactive ingredients aid in stability, bioavailability, and capsule integrity.
The formulation is carried out in Good Manufacturing Practice (GMP) certified facilities using high-precision equipment like fluid bed coaters and granulators to ensure consistency.
3. Quality Assurance and Testing
Every batch of dabigatran undergoes rigorous quality testing to ensure compliance with regulatory standards such as those set by the U.S. FDA, EMA, and other global authorities.
Testing includes:
Identity and Purity Tests: Confirm the chemical structure and absence of harmful impurities.
Dissolution Testing: Ensures the drug releases properly in the gastrointestinal tract.
Stability Studies: Long-term and accelerated testing to ensure the product remains effective over time.
Microbiological Testing: Confirms the absence of microbial contamination.
Each step in the manufacturing process is documented in detail, and full traceability is maintained from raw materials to the final packaged product.
4. Packaging and Serialization
Due to dabigatran’s sensitivity to moisture, its packaging is done in blister packs with desiccant features to ensure shelf-life stability. Additionally, in compliance with anti-counterfeit regulations, manufacturers must implement serialization—unique barcodes or digital identifiers on each unit of sale.
Packaging processes are automated and validated to ensure consistency. Labels must include all regulatory information, usage guidelines, and safety warnings.
Challenges in Dabigatran Manufacturing
Manufacturing dabigatran is far from straightforward. The primary challenges include:
Solubility and Bioavailability: Dabigatran etexilate is poorly soluble in water, making formulation a technological hurdle.
Cost of Production: Multi-step synthesis and specialized formulation techniques increase production costs.
Regulatory Scrutiny: As a high-risk medication, it falls under strict post-marketing surveillance and pharmacovigilance.
Patent and Market Competition: While originator patents have expired in many countries, producing a bioequivalent generic still requires significant investment and regulatory approval.
Global Landscape and Future Prospects
The manufacture of dabigatran has expanded globally with the rise of generic drug manufacturers. The expiration of key patents has opened up opportunities for more affordable versions, increasing global accessibility. However, ensuring consistent quality across different manufacturers remains a top priority for health regulators.
In the future, innovation may focus on improving formulations (e.g., extended-release versions), enhancing patient compliance, or integrating AI and automation into production lines for better quality control.
Conclusion
Dabigatran represents a critical advancement in anticoagulant therapy, but its availability and efficacy are rooted in a highly intricate manufacturing process. From chemical synthesis to final packaging, each stage is governed by science, precision, and stringent quality controls. As the pharmaceutical industry evolves, so too does the technology and oversight behind medications like dabigatran—ensuring patients receive safe, effective treatment every time.
URL: For more information, visit Bhasya International: Dabigatran manufacturer
0 notes
Text
CAS NO. 35180-01-9 MANUFACTURERS IN INDIA
Understanding Chloromethyl Isopropyl Carbonate (CAS No. 35180-01-9): A Key Intermediate in Pharmaceutical Synthesis
The chemical industry is the cornerstone of modern science and technology, supplying essential compounds for industries ranging from agriculture to electronics. One niche but critical segment of this industry is the manufacturing of pharmaceutical intermediates—chemical compounds used in the synthesis of active pharmaceutical ingredients (APIs). One such intermediate that plays a pivotal role in antiviral drug synthesis is Chloromethyl Isopropyl Carbonate, identified by its Chemical Abstracts Service (CAS) number: 35180-01-9.

What is Chloromethyl Isopropyl Carbonate?
Chloromethyl Isopropyl Carbonate (CMIC) is an organic compound that exists as a colorless to pale yellow liquid. It belongs to the family of chloroformates, a group of chemicals often used in organic synthesis due to their reactivity with amines, alcohols, and acids. CMIC, in particular, is prized for its role as a reagent and intermediate in the pharmaceutical sector.
Its molecular formula is C5H9ClO3, and it has a molar mass of approximately 152.58 g/mol. This unique composition imparts specific reactivity that is highly valuable in drug synthesis.
Key Applications
The primary application of Chloromethyl Isopropyl Carbonate lies in its use as a chemical intermediate in the synthesis of Tenofovir, a crucial component in antiretroviral therapy (ART). Tenofovir is used in the treatment of HIV/AIDS and Hepatitis B, diseases that affect millions worldwide. In the synthetic route for Tenofovir, CMIC is involved in the preparation of Tenofovir disoproxil fumarate (TDF), a prodrug that enhances the bioavailability of Tenofovir.
Apart from Tenofovir, CMIC may also find use in the synthesis of other nucleotide analogues and advanced pharmaceutical compounds. Because it contains both a reactive carbonate and a chloroalkyl group, it can form ester and ether bonds under controlled conditions, enabling chemists to build complex molecules.
Chemical Properties and Handling
CMIC is a reactive chemical and must be handled with care. It is moisture-sensitive and should be stored under an inert atmosphere such as nitrogen. Prolonged exposure to air and water can result in hydrolysis, which degrades the compound and reduces its effectiveness in synthetic reactions.
Here are a few key properties:
Appearance: Colorless to pale yellow liquid
Boiling Point: Approximately 140–150°C (decomposes)
Solubility: Reacts with water; soluble in common organic solvents like dichloromethane and toluene
Stability: Stable under recommended storage conditions; decomposes on exposure to moisture
Given its reactivity, personal protective equipment (PPE) is essential during handling. This includes gloves, protective eyewear, lab coats, and proper ventilation to avoid inhalation of vapors.
Role in Green Chemistry and Process Optimization
Several process improvements have been made to minimize waste, reduce the use of toxic reagents, and enhance yield. The use of CMIC in pharmaceutical synthesis is now optimized to ensure higher atom economy and better resource utilization.
Continuous flow chemistry and catalysis have also been explored to enhance the efficiency of CMIC-based processes. These advances contribute not only to economic benefits but also to the reduction of environmental footprints in pharmaceutical manufacturing.
Regulatory Considerations
As with all compounds involved in pharmaceutical synthesis, the use and transport of CMIC are subject to stringent regulations. Manufacturers must comply with:
Good Manufacturing Practices (GMP)
REACH (Registration, Evaluation, Authorization, and Restriction of Chemicals) guidelines
Environmental Health and Safety (EHS) standards
Proper documentation, batch traceability, and impurity profiling are essential for CMIC, especially when the final product is intended for human consumption.
Furthermore, CMIC is not an end-product for direct human use, but because it plays a role in life-saving drug synthesis, regulatory bodies often scrutinize the quality and purity of this intermediate. Any impurities or deviations can significantly impact the efficacy or safety of the final drug formulation.
Global Demand and Market Trends
The demand for Chloromethyl Isopropyl Carbonate is directly tied to the production of Tenofovir and similar antiviral agents. With a growing global emphasis on public health and preparedness for viral epidemics, the market for CMIC has seen steady growth. Increased awareness and access to antiretroviral therapy in low- and middle-income countries have driven the need for large-scale, cost-effective production of pharmaceutical intermediates like CMIC.
In regions like Asia—particularly India and China—CMIC is produced in bulk quantities due to the established infrastructure for API and intermediate manufacturing. These countries have positioned themselves as global suppliers, fulfilling the needs of both domestic and international pharmaceutical firms.
Safety and Environmental Impact
While CMIC is a valuable intermediate, its use does carry some environmental and health risks if not managed properly. The compound must be neutralized and disposed of according to local environmental protection guidelines. Effluent treatment plants (ETPs) and scrubber systems are typically used to minimize emissions during manufacturing.
From a workplace safety perspective, training and operational controls are essential to prevent accidental exposure. Due to its potential to irritate the respiratory tract, skin, and eyes, emergency protocols and first-aid measures must be clearly communicated and accessible.
Conclusion
Chloromethyl Isopropyl Carbonate (CAS No. 35180-01-9) may not be a household name, but its role in the pharmaceutical world is significant and indispensable. As a key intermediate in the synthesis of Tenofovir, it plays a silent yet vital role in global health by aiding the production of drugs that fight life-threatening diseases like HIV and Hepatitis B.
The chemical’s reactivity, regulatory importance, and commercial demand underscore the need for high-quality, well-controlled manufacturing processes. With ongoing research into process efficiency and environmental sustainability, the future of CMIC production is poised to support the evolving needs of the pharmaceutical industry—more safely, sustainably, and effectively than ever before.URL: For more information, visit connex pharma : CAS NO. 35180-01-9 MANUFACTURERS IN INDIA
0 notes
Text
Understanding Atovaquone Impurities: Significance in Pharmaceutical Analysis
In the pharmaceutical industry, ensuring drug safety and efficacy is non-negotiable. A critical component of this assurance lies in identifying and quantifying Atovaquone impurities — unwanted chemicals that may arise during synthesis, formulation, or storage. These impurities can affect the drug’s performance, making their identification vital for regulatory compliance and therapeutic reliability.
What Are Atovaquone Impurities?
Atovaquone, an antiprotozoal medication primarily used to treat malaria and Pneumocystis jirovecii pneumonia, must meet stringent purity requirements. Impurities can form as by-products, degradation products, or residual solvents. Identifying and controlling these impurities is essential for maintaining product quality.
Types of Atovaquone Impurities
At Aquigen BioSciences, we specialize in providing high-purity reference standards for Atovaquone impurity profiling. Some of the most commonly referenced impurities include:
Atovaquone D5 — A stable isotope-labeled compound, Atovaquone D5 is used as an internal standard in mass spectrometric analysis to enhance precision in quantifying Atovaquone and its impurities.
Atovaquone EP Impurity A — Officially listed in the European Pharmacopoeia, this impurity serves as a key marker in regulatory testing and impurity profiling.
Atovaquone EP Impurity B — Another critical reference standard for impurity testing, supporting analytical method validation and regulatory submissions.
Atovaquone EP Impurity C — Essential for advanced quality control, especially when conducting forced degradation studies and stress testing.
Why Accurate Impurity Profiling Matters
Regulatory agencies like the FDA and EMA require stringent control of drug impurities to minimize potential toxicity and ensure therapeutic consistency. Using certified impurity reference standards enables:
Regulatory compliance with pharmacopoeial standards
Enhanced analytical precision in quality control labs
Safe formulation development with minimized risk
Aquigen BioSciences: Your Trusted Partner in Impurity Standards
At Aquigen, we support pharmaceutical manufacturers, CROs, and analytical laboratories by providing high-quality impurity reference standards for Atovaquone impurities. All our products come with comprehensive COAs, including HPLC, MS, and NMR data, ensuring traceability and regulatory acceptance.
Explore our complete range of Atovaquone impurity standards here, and elevate your analytical confidence.
0 notes
Text
Here’s how companies can ensure they’re in compliance with new requirements that go into effect in August. Nitrosamine contamination has become one of the most pressing issues facing the pharmaceutical industry today. In the past few years, these potentially cancer-causing substances have grabbed the public’s attention and become a major focus of regulators, prompting the adoption of rigorous new FDA guidelines that will go into effect this summer. Since 2018, the FDA has issued more than 500 recalls because of unacceptable levels of nitrosamines in active pharmaceutical ingredients (APIs). As executive director of analytical at SK Pharmteco, I work with pharmaceutical clients to provide comprehensive testing services for a broad range of compounds, including impurities such as nitrosamines. Based on what we’re seeing, it’s clear that the spotlight on nitrosamines will only get brighter with manufacturers having to meet new FDA guidelines for these compounds by August. The new FDA guidelines, issued last year, are both comprehensive and complex. They cover more than 250 nitrosamine compounds and apply to every drug on the market or in development. These guidelines require detecting minute levels—down to the parts per billion—with limits that vary depending on the specific nitrosamine. The stakes are high for getting it right. Noncompliance triggers an FDA Class II recall, which means drugs have to be immediately pulled from shelves. The FDA will not approve new drugs unless they meet the guidelines. Compliance may necessitate reformulation of products with different excipients and inputs. Additionally, it could require redesigning production processes, as nitrosamines can form through chemical reactions between precursors that are otherwise not hazardous on their own. In light of these stakes, pharma executives must ensure they have a robust testing program in place now and are proactively addressing any issues with noncompliant nitrosamine levels. �� A Fast-Developing Issue Nitrosamines can be found in a wide range of products, from foods and beverages to cosmetics and pharmaceuticals. These compounds are created when amines react with nitrosating agents such as nitrous acid, nitrogen oxides and nitrites. Nitrosamines were observed by chemists in the late 1800s, but the dangers were not understood at the time. In the 1970s, William Lijinsky, laboratory director at the National Cancer Institute’s Frederick Cancer Research and Development Facility, became a leading voice in raising concerns about their link to cancer. Initial concerns focused on tobacco products and foods, such as processed meats, where smoking or curing can introduce nitrites to trigger the chemical reaction. In the following decades, concerns grew as more studies indicated a link between different nitrosamines and cancer. Studies also showed that even small amounts of nitrosamines could be dangerous, leading regulators and experts to place greater scrutiny on their presence in products such as pharmaceuticals. In 2018, the FDA began an investigation into nitrosamine impurities in drugs that led to high-profile recalls for heartburn medicines. In 2020, the FDA issued its first guidance on the compounds, making nitrosamines an issue for the entire pharmaceutical industry for the first time. The FDA’s actions corresponded with increasing public concern about potential carcinogens in everything from food to toys and even water. While deaths from cancer have declined in the U.S., the number of new cases continues to climb each year, according to the American Cancer Society. The existence of nitrosamines in prescription drugs is particularly troubling because the medications are intended to treat illnesses, not cause them. They’re also intended to be taken on a regular basis. The FDA’s rapid and comprehensive response to the nitrosamine issue signals that it has taken a place among the agency’s top priorities in protecting the public. SK Pharmteco has seen pharma’s quick response to this change. ...
0 notes
Text
Trusted Tirofiban Manufacturer For High-Quality APIs
Tirofiban Manufacturer is a famous antiplatelet drug used in the manipulation of acute coronary syndrome (ACS). As a non-peptide glycoprotein IIb/IIIa inhibitor, it prevents platelet aggregation and is essential subsequently for percutaneous coronary interventions (PCI). With its growing call for in cardiology and emergency remedy, sourcing Tirofiban from a reliable and GMP-compliant producer is vital for pharmaceutical corporations, hospitals, and studies establishments.
What Is Tirofiban?
Tirofiban is normally to be had as Tirofiban hydrochloride monohydrate, a white to off-white powder soluble in water. It’s formulated into injectable answers used to save you blood clots in sufferers with coronary coronary coronary heart conditions. The drug works via inhibiting fibrinogen binding to platelet receptors, thereby lowering the hazard of clot-related sports activities collectively with coronary coronary coronary coronary coronary heart attacks.
Key Considerations When Choosing a Tirofiban Manufacturer
1. Regulatory Compliance and Certifications
The most important trouble at the same time as deciding on a Tirofiban Manufacturer in China is compliance with global pharmaceutical requirements. Look for businesses that take a look at Good Manufacturing Practices (GMP) and characteristic certifications which includes WHO-GMP, US FDA, EU GMP, or ISO 9001. These certifications make certain the producing environment is sterile, controlled, and meets terrific requirements critical for human-use prescribed drugs.
2. API and Formulation Capabilities
Some producers' reputation certainly on producing the Active Pharmaceutical Ingredient (API), at the same time as others moreover offer formulated injectables. Depending to your goals, choose a manufacturer that offers the famous product form at the facet of precise documentation collectively with the Certificate of Analysis (COA), Drug Master File (DMF), and Stability Data.
3. Research and Development Support
For pharmaceutical organizations growing new cardiovascular treatment options, having R&D guidance from the manufacturer is a primary advantage. Leading producers regularly provide custom synthesis, stability research, and device development offerings. This partnership can boost up scientific trials and regulatory submissions.
4. Quality Control and Assurance
A reliable Tirofiban Manufacturer can also have in-residence analytical laboratories to carry out rigorous trials out of every batch. This consists of exams for impurities, balance, and performance. Make terrific they comply with ICH suggestions and provide batch records and complete traceability.
5. Global Supply and Logistics
Tirofiban is a sensitive drug that calls for proper cold chain logistics and ordinary packaging. A specific producer ought to provide inexperienced export services, help with regulatory filings, and a robust logistics community to make sure properly timed deliveries ultimately of regions. Local warehousing alternatives additionally may be useful for consistent delivery.
6. Pricing and Transparency
While price is a problem, in no manner compromise is incredible. Be careful of alternatively low expenses as they will advocate compromised manufacturing practices or loss of regulatory compliance. Always request samples, validate the COA, and conduct audits or 0.33-celebration inspections if crucial.
Final Thoughts
Finding a reliable Tirofiban Manufacturer China consists of extra than evaluating expenses. Quality, compliance, documentation, and issuer help are essential at the identical time as sourcing this form of essential cardiovascular drug. Whether you are a pharmaceutical commercial enterprise organisation or a medical institution procurement crew, making an funding time in vetting your corporation will ensure affected person safety and regulatory peace of thoughts.
0 notes
Text
Manufacturing Nilotinib API: Key Process Considerations and Quality Challenges

Nilotinib is a second-generation tyrosine kinase inhibitor (TKI) primarily used in the treatment of chronic myeloid leukemia (CML). As an active pharmaceutical ingredient (API), nilotinib plays a crucial role in inhibiting the BCR-ABL protein that causes the uncontrolled proliferation of leukemic cells. Given its therapeutic importance and stringent safety requirements, manufacturing nilotinib API demands meticulous planning, process control, and regulatory compliance.
This article explores the key considerations in the production of nilotinib API, along with the major quality challenges that manufacturers must navigate to ensure safe and effective delivery to end-users.
1. Synthetic Route Development
The manufacturing of nilotinib begins with route selection, which directly impacts yield, cost-efficiency, and impurity formation. The synthesis process typically involves multiple steps, including heterocyclic coupling, amine protection/deprotection, and amide bond formation.
Process developers must evaluate several critical parameters during route design:
Reagent selection and cost
Reaction efficiency and scalability
Environmental and safety factors
Control of side reactions and by-products
Establishing a high-purity intermediate pathway is essential to minimize downstream purification load and reduce the risk of impurities exceeding ICH limits.
2. Impurity Profiling and Control
One of the most complex aspects of nilotinib API production is impurity identification and control. Because the molecule contains sensitive functional groups and multiple chiral centers, it is prone to the formation of related substances, isomers, and degradation products.
To address this, manufacturers must:
Implement robust analytical methods (e.g., HPLC, LC-MS) for detection and quantification
Set strict specification limits based on toxicological thresholds
Perform comprehensive forced degradation studies to anticipate and control impurities under various storage and processing conditions
Impurities must be classified and qualified according to ICH Q3A/B guidelines to ensure product safety.
3. Process Validation and GMP Compliance
Nilotinib API falls under high-value, regulated pharmaceutical ingredients, and as such, requires full Good Manufacturing Practice (GMP) compliance. The entire process—from raw material sourcing to final API crystallization—must be thoroughly validated.
Key areas of focus during validation include:
Critical Process Parameters (CPPs): Temperature, pH, solvent ratios
Critical Quality Attributes (CQAs): Assay, purity, residual solvents, particle size
Equipment qualification and cleanroom standards to prevent contamination
Manufacturers are required to maintain detailed batch manufacturing records (BMRs) and validation master plans (VMPs), especially if the API is intended for regulated markets like the US, EU, or Japan.
4. Polymorphism and Crystallization Control
The solid-state properties of nilotinib play a critical role in its bioavailability, solubility, and stability. During final crystallization, controlling polymorphic form is essential to maintain consistency in performance and comply with regulatory filings.
Challenges here include:
Identifying and maintaining the correct crystal form (e.g., Form A, Form B)
Avoiding unintended form conversion during drying or storage
Optimizing solvent selection and cooling profiles
Failure to maintain a consistent polymorphic profile can lead to regulatory rejections and batch failures in downstream formulation.
5. Environmental and Safety Considerations
The use of organic solvents, reagents like phosphorylating agents, and elevated reaction temperatures make nilotinib synthesis both environmentally and operationally sensitive. Manufacturers must ensure:
Efficient waste management and solvent recovery systems
Compliance with EHS (Environment, Health, and Safety) guidelines
Worker safety through proper PPE, closed systems, and process automation
Minimizing the ecological footprint while maximizing yield remains a balancing act in nilotinib API production.
Conclusion
The manufacturing of nilotinib API is a highly specialized process that involves a delicate interplay of synthetic chemistry, regulatory rigor, and quality assurance. From route development and impurity control to polymorphic consistency and GMP compliance, every step must be optimized for safety, scalability, and global regulatory acceptance.
As demand for high-performance oncology APIs grows, nilotinib API manufacturers that focus on innovation, quality control, and environmental responsibility will remain critical to the success of global pharmaceutical supply chains.
0 notes
Text
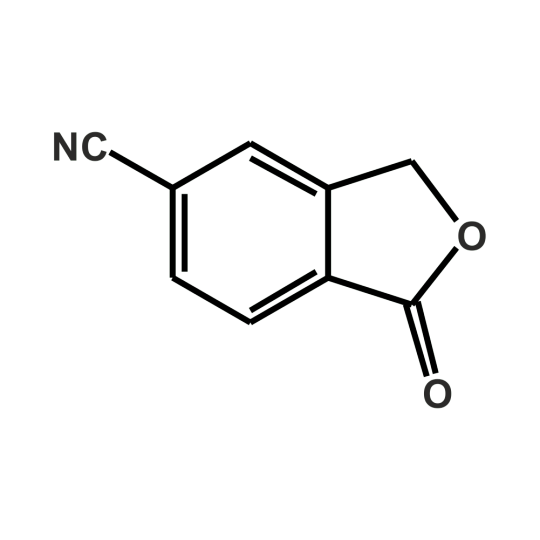
Exploring 5-Cyanophthalide: Key Intermediate Backed by Jay Finechem’s Expertise
In the ever-evolving pharmaceutical and fine chemicals industry, precision, purity, and performance are critical. One such essential compound that stands out as a versatile intermediate is 5-Cyanophthalide. Widely used in the synthesis of complex pharmaceutical compounds, including Citalopram KSM, 5-Cyanophthalide plays a pivotal role in streamlining advanced manufacturing processes. With increasing global demand for high-quality Phthalide derivatives, sourcing this intermediate from a trusted, experienced supplier becomes paramount.
Enter Jay Finechem, a leading 5-Cyanophthalide manufacturer known for quality, compliance, and reliability. This blog delves into the science, applications, and sourcing of 5-Cyanophthalide, with a spotlight on why Jay Finechem is the preferred partner for global buyers looking for 5-Cyanophthalide in India and beyond.
What is 5-Cyanophthalide?
5-Cyanophthalide, identified by CAS 82104-74-3, is a chemical intermediate classified under Phthalide derivatives. It has a unique structure comprising a phthalide ring substituted with a nitrile (-CN) group at the fifth position, making it chemically stable and suitable for further transformation in synthetic pathways.
Molecular Formula: C9H5NO2
Molecular Weight: 159.14 g/mol
IUPAC Name: 3-Oxo-1,3-dihydroisobenzofuran-5-carbonitrile
CAS Registry: CAS 82104-74-3
The compound is typically found as a white to off-white crystalline powder and must be stored under controlled conditions to maintain its chemical integrity. Its structure lends it great value in organic synthesis, particularly in producing Citalopram KSM, an essential active pharmaceutical ingredient intermediate.
Properties and Chemical Characteristics
5-Cyanophthalide exhibits properties that make it ideal for pharmaceutical and specialty chemical applications:
Thermal Stability: It remains stable under moderate heat and ambient conditions.
Solubility: Soluble in polar organic solvents such as DMSO, DMF, and acetonitrile.
Reactivity: The nitrile group (-CN) at the 5th position facilitates further reactions like hydrolysis, reduction, and amidation.
These characteristics make 5-Cyanophthalide a sought-after intermediate in both large-scale and custom synthesis operations. When synthesized under stringent conditions by a qualified 5-Cyanophthalide manufacturer, the compound achieves exceptional levels of purity, often exceeding 98%, crucial for regulated pharmaceutical processes.
Applications: Where 5-Cyanophthalide Shines
The primary application of 5-Cyanophthalide is as an intermediate in the synthesis of high-value pharmaceutical compounds. Its most prominent role is in the manufacture of Citalopram KSM, a critical precursor for Citalopram, an SSRI used globally to treat depression and anxiety.
Major Uses:
API Manufacturing: Especially in the synthesis of Citalopram KSM.
Custom Pharma Synthesis: Enables modular chemical transformations.
R&D in Specialty Chemicals: Used in research labs developing novel Phthalide derivatives.
Its versatility also extends to fine chemical manufacturing, where companies use it as a base to develop structurally similar compounds with pharmacological relevance. This has made 5-Cyanophthalide in India a keyword of growing interest among international buyers seeking reliable sources.
The Importance of Purity in 5-Cyanophthalide
High-purity 5-Cyanophthalide is essential for pharmaceutical applications. Even minute impurities can affect the efficacy and safety of the final API, leading to regulatory issues and reduced product performance. That’s why leading companies rely on verified 5-Cyanophthalide suppliers like Jay Finechem who offer:
GMP-compliant production
Batch-wise quality reports (COA)
Analytical support (HPLC, NMR, GC-MS)
For pharmaceutical companies, sourcing from a certified 5-Cyanophthalide manufacturer ensures that the intermediate meets international standards required for drug registration and regulatory audits. In a landscape where quality equals compliance, consistent supply from reputable partners is a business-critical decision.
Why Jay Finechem is a Preferred 5-Cyanophthalide Manufacturer
Jay Finechem has built a strong reputation in the fine chemicals industry, especially for producing high-purity intermediates like 5-Cyanophthalide. With decades of experience and an uncompromising focus on quality, the company is recognized as a go-to 5-Cyanophthalide supplier for both domestic and international markets.
What Sets Jay Finechem Apart?
State-of-the-art Manufacturing: Facilities equipped for large-scale and small-batch production.
Regulatory Compliance: Follows cGMP, ISO, and other international norms.
Technical Expertise: In-house R&D team for process optimization and innovation.
Customer Support: End-to-end support from quotation to post-delivery documentation.
Being a leading 5-Cyanophthalide manufacturer in India, Jay Finechem caters to a wide variety of industries ranging from pharma to agrochemicals, providing reliable access to one of the most important Phthalide derivatives on the market.
5-Cyanophthalide in India: Why the Country is a Global Hub
The production of 5-Cyanophthalide in India has gained global traction due to the country’s robust chemical manufacturing ecosystem. India offers:
Cost-effective synthesis
Highly skilled chemical workforce
Export-friendly regulations
Rich vendor base for raw materials
With many global buyers now focusing on “China +1” sourcing strategies, India has emerged as a reliable and scalable supplier. Jay Finechem, headquartered in this growing ecosystem, takes full advantage of India’s infrastructure to serve clients worldwide.
If you're searching for CAS 82104-74-3 in India, Jay Finechem's capabilities in handling orders of any scale, along with timely global logistics, make it an ideal choice.
CAS 82104-74-3: Understanding the Importance of Chemical Registry Numbers
In the world of fine and specialty chemicals, CAS numbers offer a universal identification method for substances. CAS 82104-74-3 is the unique identifier for 5-Cyanophthalide, enabling regulatory compliance, inventory management, and accurate procurement.
Sourcing through this identifier ensures:
Avoidance of isomer confusion
Correct documentation for quality and compliance
Precise product tracking across international borders
Jay Finechem ensures that every shipment is properly labeled with CAS 82104-74-3, along with required safety and quality documentation, which is especially critical for clients working under strict regulatory oversight.
Jay Finechem’s Edge as a Global 5-Cyanophthalide Supplier
Jay Finechem doesn’t just manufacture chemicals — it provides dependable chemical solutions. As a trusted 5-Cyanophthalide supplier, the company brings several competitive advantages to the table:
Key Differentiators:
Global Reach: Exporting to Europe, North America, and Asia.
Custom Synthesis: Tailored processes for client-specific needs.
End-to-End Documentation: COA, MSDS, and compliance certificates included.
Environmental Responsibility: Eco-friendly processes with minimized waste.
Whether your requirement is for gram-scale R&D or metric tons for bulk manufacturing, Jay Finechem delivers with consistency and confidence. When sourcing 5-Cyanophthalide in India, few names match Jay Finechem in terms of quality, transparency, and customer care.
Conclusion: Your Trusted Partner for 5-Cyanophthalide and Beyond
From its critical role in synthesizing Citalopram KSM to being a key player in producing Phthalide derivatives, 5-Cyanophthalide continues to shape innovation in pharmaceuticals and fine chemicals. With rising global demand, choosing the right partner for sourcing this intermediate is more important than ever.
Jay Finechem stands out as a highly qualified 5-Cyanophthalide manufacturer and supplier, offering unmatched quality, regulatory support, and global reach. If you're looking to procure 5-Cyanophthalide (CAS 82104-74-3) from a reliable source with proven expertise, Jay Finechem is your ideal partner.
0 notes
Text
Understanding the Uses of Hyoscine Hydro Bromide in Medicine – Prism Industries Pvt. Ltd.
In today's world of pharmaceuticals, some compounds have stood the test of time, proving their worth through generations of clinical use. One such compound is hyoscine hydro bromide, a well-known anticholinergic agent. At Prism Industries Pvt. Ltd., we take great pride in being a trusted partner in the API manufacturing sector, delivering top-notch hyoscine hydro bromide for a variety of therapeutic applications. With our commitment to precision, quality, and tailored API manufacturing, we ensure that healthcare systems around the globe receive APIs they can rely on.
What is Hyoscine Hydro Bromide?
Hyoscine hydro bromide, often referred to as scopolamine hydrobromide, is a medication designed to tackle motion sickness, nausea, vomiting, and gastrointestinal issues stemming from muscle spasms. It operates by blocking specific neurotransmitters in the nervous system, particularly acetylcholine, which plays a key role in regulating smooth muscle contractions.
This compound is derived from the Solanaceae plant family and is synthesized and purified under rigorous quality standards to comply with international pharmacopeial guidelines.
Key Therapeutic Uses of Hyoscine Hydro Bromide
Motion Sickness Prevention: One of its most popular applications, hyoscine hydro bromide is highly effective in preventing and managing nausea, vomiting, and dizziness associated with motion or travel.
Post-Operative Nausea: It's frequently administered in hospitals to help alleviate nausea and vomiting after surgical procedures.
Irritable Bowel Syndrome (IBS): Thanks to its antispasmodic properties, hyoscine hydro bromide can ease cramping and abdominal pain linked to IBS.
Parkinson’s Disease Support: It’s often used alongside other treatments to help manage symptoms like drooling and stiffness.
Gastrointestinal Disorders: This compound is commonly found in formulations aimed at relieving colic, indigestion, and other related spasmodic conditions.
Prism Industries – A Trusted Leader in API Manufacturing
When it comes to API manufacturing, Prism Industries Pvt. Ltd. stands out as a respected name in the industry. We provide pharmaceutical ingredients that not only meet but exceed global regulatory and quality standards. Our process for manufacturing hyoscine hydro bromide is validated, and we carry out thorough quality checks at every step of production.
Our facilities are GMP-certified and boast state-of-the-art infrastructure, ensuring consistency and reproducibility in every batch. From sourcing raw materials to the final packaging of APIs, we prioritize transparency and traceability throughout the entire process.
Why Choose Hyoscine Hydro Bromide from Prism Industries?
- Exceptional purity and consistent potency
- Options for customizable particle sizes and bulk packaging
- Rigorous impurity profiling and stability testing
- Fully compliant with EP, BP, and IP standards
Diverse Applications in the Medical Field
Hyoscine hydro bromide is incredibly versatile, making it a key ingredient in a wide range of pharmaceutical formulations, including:
- Oral tablets and chewable forms
- Injectable solutions
- Transdermal patches (particularly effective for travel sickness)
- Pediatric syrups
- Rapid-action suppositories
We partner with pharmaceutical companies around the world, offering custom API manufacturing solutions that cater to their unique product development requirements.
Regulatory Support and Documentation
We’re here to help our global clients with a comprehensive range of documentation that makes product registration and regulatory filing a breeze:
- Drug Master File (DMF)
- Certificate of Analysis (CoA)
- Stability Data
- MSDS and Technical Specifications
Our skilled regulatory team is also on hand to provide dossier support, helping clients secure faster approvals in both regulated and semi-regulated markets.
Customization in API Supply
Every pharmaceutical brand has its own unique requirements. At Prism Industries Pvt. Ltd., we deliver tailored API manufacturing solutions from start to finish:
- Bulk or small-batch production
- Controlled release-ready API grades
- Flexible packaging sizes and labeling
- Customized impurity profiles for optimizing formulations
Our technical team collaborates closely with formulation scientists and R&D teams to create solutions that boost performance and enhance market competitiveness.
Sustainability and Safety in Production
As a responsible participant in the API manufacturing sector, we place a strong emphasis on safety, sustainability, and environmental stewardship. Our production of hyoscine hydro bromide adheres to:
- Green chemistry protocols
- Waste minimization and solvent recycling
- Zero-discharge effluent management
- Safe and hygienic working conditions
These practices not only help us meet regulatory standards but also contribute to reducing our carbon footprint.
Prism Industries is a trusted global player in the API manufacturing industry, serving clients in over 40 countries. Our hyoscine hydro bromide is relied upon by leading pharmaceutical companies around the world, from the UK and Germany to South Africa, Egypt, Brazil, the Philippines, Vietnam, and India. Thanks to our robust logistics support, we ensure safe, timely, and regulatory-compliant delivery of APIs across the globe.
As a specialized manufacturer of plant-derived and synthetic antispasmodics, we take pride in our decades of expertise in the API industry. Our regulatory documentation and global compliance give our partners the confidence they need. We also provide tailored solutions to support their formulation innovation, with a customer-centric approach that prioritizes prompt response, transparent communication, and reliable supply chains.
Looking ahead, we continue to invest in research and process development to explore newer applications of hyoscine hydro bromide. This includes enhanced delivery systems, combination APIs for targeted therapies, and pediatric and geriatric-friendly formulations. As a trusted name in the industry, Prism Industries is committed to empowering global healthcare through quality, compliance, and customization.
#hyoscine_hydro_Bromide#hyoscine_butyl_bromide_uses#hyoscine_hydrobromide#nicotine_Manufacturer#api_manufacturing_services
0 notes
Text
Best Hydrochloric Acid 32% Manufacturer in Philippines

Introduction
The pharmaceutical and chemical industries in the Philippines are rapidly growing, with increasing demand for high-quality raw materials and chemical compounds. Among the most essential industrial chemicals is Hydrochloric Acid 32%, a versatile and widely used compound in manufacturing, pharmaceuticals, food processing, and water treatment. Finding a reliable and high-purity manufacturer is crucial for industries that require consistency, safety, and compliance.
If you're looking for the best Hydrochloric Acid 32% manufacturer in the Philippines, Dolphin Pharmaceutical is a name you can trust. As a leading pharmaceutical drug manufacturer in Philippines, the company is known for delivering world-class chemical products backed by international quality standards and excellent customer service.
Understanding Hydrochloric Acid 32%
Hydrochloric Acid (HCl) is a colorless to slightly yellow liquid with a sharp, pungent odor. When diluted to a 32% concentration, it becomes a highly effective solution used in multiple applications:
pH regulation in pharmaceuticals and water treatment
Cleaning and pickling of metals in the steel industry
Production of organic compounds
Food processing (as an acidifier)
Oil well acidizing in petroleum industries
Its utility across sectors makes Hydrochloric Acid a chemical in constant demand, especially when manufactured under stringent safety and quality standards.
Why Quality Matters
Quality matters most when dealing with a highly reactive substance like HCl. Impurities or inconsistency in concentration can affect downstream processes and pose safety hazards. Therefore, industries seek a manufacturer that provides:
High purity (minimum 32% concentration)
Safe and compliant packaging
Accurate documentation
Consistent supply and logistics support
Dolphin Pharmaceutical ensures all these, making it the top choice for companies in need of Hydrochloric Acid 32% in the Philippines.
Dolphin Pharmaceutical: Your Trusted Manufacturing Partner
As a leading pharmaceutical drug manufacturer in Philippines, Dolphin Pharmaceutical doesn’t just focus on medicine — it also provides crucial raw materials like Hydrochloric Acid 32%. The company has invested in modern infrastructure, trained professionals, and global quality certifications to ensure that every product meets international benchmarks.
Key Features:
GMP-Compliant Plants: Our production facilities follow Good Manufacturing Practices and adhere to environmental safety norms.
Advanced Testing Labs: Each batch is rigorously tested for purity, concentration, and contamination before dispatch.
Customized Packaging: We offer safe and customizable packaging solutions that meet industrial safety norms and client needs.
On-Time Delivery: With a robust distribution network across the Philippines, we ensure timely deliveries to all regions.

Applications in the Pharmaceutical Industry
Hydrochloric Acid 32% plays a critical role in drug formulation, especially for pH balance, synthesis of APIs (Active Pharmaceutical Ingredients), and cleaning equipment. Pharmaceutical companies rely on HCl to ensure sterility, process accuracy, and regulatory compliance.
Being both a Hydrochloric Acid manufacturer and a pharmaceutical drug manufacturer in Philippines, Dolphin Pharmaceutical uniquely understands the standards required for pharmaceutical applications — offering dual expertise that adds unmatched value to clients.
Environmental and Safety Commitment
Handling acids like HCl requires care, and Dolphin Pharmaceutical takes its environmental responsibilities seriously. Our safety protocols include:
Leak-proof containers and packaging
Proper labeling and transport compliance
Employee safety training and audits
Effluent treatment and emission control systems
This commitment not only protects people and the environment but also builds long-term trust with our clients.
Industries We Serve
While pharmaceutical companies form a core client base, our Hydrochloric Acid 32% also serves the following sectors:
Food & Beverage: Used for food-grade applications and sanitation
Textile: Dyeing, bleaching, and processing textiles
Metal Processing: Rust removal and surface treatment
Water Treatment: Balancing water pH in industrial systems
Oil & Gas: Acidizing oil wells for improved extraction
Our clients include top manufacturers, hospitals, laboratories, and exporters throughout the Philippines and abroad.
Why Choose Dolphin Pharmaceutical
Here’s why customers consider us the best Hydrochloric Acid 32% manufacturer in the Philippines:
Decade-long expertise in chemical and pharmaceutical manufacturing
Global certifications like ISO, WHO-GMP, and local regulatory approvals
Tailored solutions for small-scale and large-scale requirements
Affordable pricing with high-grade quality assurance
Proven track record with multinational and local clients
Conclusion
Choosing the right manufacturer for Hydrochloric Acid 32% is not just about cost — it’s about quality, safety, consistency, and service. Dolphin Pharmaceutical brings all these strengths to the table, along with the credibility of being a trusted pharmaceutical drug manufacturer in Philippines.
Whether you are in the pharmaceutical, food, water treatment, or metal industry, Dolphin Pharmaceutical ensures that your HCl requirements are met with the highest level of precision and professionalism.
#Hydrochloric Acid 32% Manufacturer in Philippines#Hydrochloric Acid 32% supplier in Philippines#Hydrochloric Acid 32% exporter in Philippines
0 notes
Text
The Importance of Material Testing Labs for UAE’s Oil and Gas Industry | +971 554747210
The United Arab Emirates (UAE) stands as one of the world’s leading producers and exporters of oil and gas, with the sector playing a critical role in the nation's economy. In such a high-stakes industry, safety, durability, and efficiency are non-negotiable. This is where Material Testing Lab come into the picture — serving as indispensable partners that help maintain the integrity of infrastructure, equipment, and processes in the oil and gas industry.
In this blog, we explore the vital importance of material testing labs for the UAE’s oil and gas sector, highlighting how these facilities ensure quality, safety, and compliance in one of the most challenging industrial environments.
Why Material Testing is Crucial in the Oil and Gas Industry
Oil and gas operations are characterized by extreme conditions — high pressures, corrosive environments, fluctuating temperatures, and mechanical stresses. Components such as pipelines, drilling equipment, storage tanks, and refinery machinery must withstand these challenges to prevent failures, leaks, or catastrophic accidents.
Material testing labs evaluate the properties and performance of raw materials and finished products to ensure they meet rigorous industry standards. This proactive approach prevents costly downtime, environmental hazards, and loss of life.
Key Roles of Material Testing Labs in the UAE Oil and Gas Sector
1. Ensuring Material Quality and Compliance
Material testing labs verify that the materials used — steel, alloys, composites, coatings, and more — conform to industry-specific standards like API (American Petroleum Institute), ASTM, ISO, and BS standards. These standards dictate chemical composition, mechanical strength, corrosion resistance, and durability.
In the UAE, local regulations and international oil companies’ specifications require stringent quality checks. Accredited labs provide unbiased, accurate test results that demonstrate compliance and enable smooth regulatory approvals.
2. Corrosion Testing and Prevention
Corrosion is one of the biggest threats to oil and gas infrastructure, especially in the UAE’s harsh desert and marine environments. Material testing labs conduct specialized corrosion testing, including salt spray tests, electrochemical analysis, and accelerated aging tests.
These tests help identify vulnerable materials or protective coatings, enabling operators to select corrosion-resistant options or schedule preventive maintenance, thereby extending the lifespan of assets.
3. Mechanical and Structural Testing
Oil and gas equipment must withstand intense mechanical loads and stresses. Material testing labs perform mechanical tests such as tensile strength, hardness, impact resistance, and fatigue testing to assess how materials behave under pressure and strain.
Structural components like pipes, valves, and pressure vessels undergo rigorous testing to certify their ability to handle operational demands, avoiding failures that could lead to spills or explosions.
4. Weld Testing and Inspection
Welding is fundamental in constructing pipelines and refinery equipment. Defects in welds can cause leaks or structural failures. Material testing labs carry out non-destructive testing (NDT) methods — including ultrasonic testing, radiography, magnetic particle inspection, and dye penetrant testing — to detect cracks, voids, and inconsistencies in welds.
Reliable weld inspection ensures the safety and longevity of welded joints critical to oil and gas operations.
5. Chemical and Material Composition Analysis
Understanding the exact chemical makeup of materials is essential to predict their behavior under operational conditions. Labs conduct chemical analyses using spectroscopy, chromatography, and other advanced techniques to detect impurities or verify alloy compositions.
This helps avoid material incompatibilities that could lead to corrosion, embrittlement, or other failures in the demanding oil and gas environment.
6. Testing for Safety and Environmental Compliance
With increasing focus on environmental protection and workplace safety, material testing labs assist oil and gas companies in meeting local and international safety regulations. Tests related to fire resistance, toxicity, and environmental impact of materials and coatings help ensure safer operations and reduce the ecological footprint.
In the UAE, adherence to standards set by authorities like the Abu Dhabi Environment Agency and Dubai Municipality is critical, and labs provide the necessary certification.
Benefits of Using Material Testing Labs for the UAE Oil and Gas Industry
1. Risk Mitigation
Material failures in oil and gas projects can lead to accidents, injuries, and costly shutdowns. Regular and rigorous material testing identifies potential issues early, reducing the risk of accidents and enhancing overall safety.
2. Cost Savings
While testing involves upfront costs, it saves money in the long term by preventing equipment failure, extending asset life, and reducing maintenance frequency. Quality assurance through labs also avoids project delays due to non-compliance or substandard materials.
3. Regulatory Compliance
Operating within UAE’s regulatory framework is essential to avoid fines and legal challenges. Material testing labs provide the documentation and certifications required to demonstrate compliance with safety and environmental laws.
4. Enhanced Product and Project Quality
Material testing labs contribute to higher quality projects by verifying that only materials meeting the highest standards are used. This improves operational efficiency, reliability, and client confidence.
5. Supporting Innovation and Sustainable Practices
With the UAE’s push toward sustainable energy and technology, material testing labs assist in evaluating new materials and technologies for enhanced performance and reduced environmental impact, supporting innovation in the oil and gas sector.
Choosing the Right Material Testing Lab for Oil and Gas Projects in the UAE
Given the critical role of material testing labs, selecting the right partner is crucial. Here are important criteria:
Accreditation and Certification: Look for labs accredited by ISO/IEC 17025 and recognized by UAE regulatory bodies.
Industry Experience: Choose labs with proven expertise in oil and gas material testing.
Comprehensive Testing Services: Ensure the lab offers mechanical, chemical, corrosion, NDT, and environmental testing.
State-of-the-Art Equipment: Advanced testing technology is key for reliable results.
Timely Delivery: Fast turnaround times help keep projects on schedule.
Clear Reporting: Detailed, transparent test reports with recommendations support informed decision-making.
Conclusion
The oil and gas industry is the backbone of the UAE’s economy, and ensuring the safety and quality of materials used is paramount. Material Testing Labs play an indispensable role by providing scientific testing, analysis, and certification that safeguard infrastructure and personnel.
By choosing accredited, experienced labs with comprehensive services, oil and gas companies in the UAE can mitigate risks, ensure compliance, reduce costs, and enhance project quality. In an industry where failure is not an option, material testing labs offer the assurance and reliability that modern oil and gas operations demand.
0 notes